Tuesday, June 19, 2012
Kocher criteria for pediatric septic hip
The Kocher criteria are useful for distinguishing between septic arthritis and transient synovitis in a child presenting with a painful hip. The criteria are as follows:
1. Non-weight-bearing on affected side
2. ESR > 40 mm/hr
3. Fever
4. Serum WBC > 12,000
Criteria met and probability of septic arthritis:
0/4 0.3%
1/4 3%
2/4 40%
3/4 93%
4/4 99%
References:
1. Kocher MS, Zurakowski D, Kasser JR> Differentiating between septic arthritis and transient synovitis of the hip in children: An evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81:1662-70.
How to insert an LMA
How to put in an LMA:
A Laryngeal mask airway, or LMA, is a supraglottic airway device. An LMA is a great rescue airway when you encounter difficulty in intubating a patient. It can also be used to as an easy and more effective alternative to bag-valve mask ventilation.
To insert an LMA:
1. Make sure the device is deflated.
2. Apply lubricant to the anterior and posterior surfaces of the LMA.
3. Standing at the head of the bed, hold the device in the dominant hand and open the airway (jaw-thrust is helpful to get tongue out of the way). Tip of index finger should rest on cuff-tube junction.
4. Point the tip of the LMA at the hard palate and advance along the hard and soft palates using a circular motion.
5. Stop when resistance is met and only the tube is protruding from the mouth.
6. Inflate the cuff. Proper placement is confirmed by ability to ventilate the patient, end-tidal CO2 return, and seeing the tube elevate slightly when cuff is inflated.
A Laryngeal mask airway, or LMA, is a supraglottic airway device. An LMA is a great rescue airway when you encounter difficulty in intubating a patient. It can also be used to as an easy and more effective alternative to bag-valve mask ventilation.
To insert an LMA:
1. Make sure the device is deflated.
2. Apply lubricant to the anterior and posterior surfaces of the LMA.
3. Standing at the head of the bed, hold the device in the dominant hand and open the airway (jaw-thrust is helpful to get tongue out of the way). Tip of index finger should rest on cuff-tube junction.
4. Point the tip of the LMA at the hard palate and advance along the hard and soft palates using a circular motion.
5. Stop when resistance is met and only the tube is protruding from the mouth.
6. Inflate the cuff. Proper placement is confirmed by ability to ventilate the patient, end-tidal CO2 return, and seeing the tube elevate slightly when cuff is inflated.
Saturday, March 3, 2012
Prevention of desaturation during intubations
A great article in this month's Annals of Emergency Medicine from Scott Weingart and Richard Levitan: "Preoxygenation and Prevention of Desaturation During Emergency Airway Management."
Recommendations for improving oxygenation and preventing desaturation during ED intubations:
1. Preoxygenate patient using standard reservoir facemask with highest possible flow rate of O2, head-up position, when possible.
2. If possible, preoxygenate for 3 minutes or have patient take 8 maximal inhalation/exhalation breaths.
3. For patients who cannot achieve saturations > 93-95% with high FiO2, consider preoxygenation with PEEP, including CPAP masks, noninvasive positive-pressure ventilation, or PEEP valves on a bag-valve-mask device.
4. Provide passive oxygenation during RSI (using high-flow O2 via nasal cannula after sedatives and paralytics given) to increase duration of safe apnea in ED tracheal intubations.
6. Ventilate hypoxemic patients during onset phase of muscle relaxants in RSI prior to tracheal intubation. For fully oxygenated patients at low risk for desaturation, ventilation is not required during the onset phase of muscle relaxants.
7. Position patients to maximize upper airway patency using ear to sternal notch positioning.
References:
1. Weingart, S and Levitan, R. Preoxygenation and Prevention of Desaturation During Emergency Airway Management. Annals of Emergency Medicine 2012;59:165-75.
References:
1. Weingart, S and Levitan, R. Preoxygenation and Prevention of Desaturation During Emergency Airway Management. Annals of Emergency Medicine 2012;59:165-75.
Normal ESR values by age
Rule for correcting erythrocyte sedimentation rate (ESR) by age:
Men: Age in years / 2
Women: Age in years + 10 / 2
References:
1. Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. BMJ 1983; 286:266.
Men: Age in years / 2
Women: Age in years + 10 / 2
References:
1. Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. BMJ 1983; 286:266.
What are the top causes of small bowel obstruction?
 |
| Upright abdominal film showing multiple air-fluid levels. |
#1. Adhesions after abdominal surgery (60%)
#2. Malignancy (20%)
#3. Hernia (10%)
#4. Inflammatory bowel disease (5%)
Intussusception is the most common cause of small bowel obstruction in children.
References:
1. Nobie, BA. Small-Bowel Obstruction. Emedicine. Available at http://emedicine.medscape.com/article/774140-overview#aw2aab6b2b3. Accessed March 3, 2012.
Thursday, February 9, 2012
What are appropriate antibiotics for PID?
Pelvic inflammatory disease (PID), refers to infection and inflammation of the female genital tract, including endometritis, salpingitis, tubo-ovarian abscess, and pelvic peritonitis. PID is believed to be initiated by ascending infection from the vagina and cervix (often Chlamydia trachomatis), but is often polymicrobial.
The CDC recommends empiric treatment for PID in sexually active young women and other women at risk for STDs if no cause for symptoms can be found and the patient has at least one of the following:
1. Cervical motion tenderness
OR
2. Adnexal tenderness
OR
3. Uterine tenderness
The following criteria enhance specificity and further support the diagnosis of PID:
1. Temp > 101 F
2. Abnormal mucopurulent cervical or vaginal discharge
3. Presence of abundant leukocytes on wet prep
4. Elevated ESR
5. Elevated CRP
6. Laboratory evidence of C. trachomatis or N. gonorrhea cervical infection
For patients with mild to moderate PID who can be managed as outpatients, the CDC recommends:
Patient who don't respond to oral therapy within 72 hours should be reevaluated both to confirm the diagnosis and to initiate parenteral therapy.
For patients with severe illness, tubo-ovarian abscess, pregnancy, or lack of response to oral agents, the CDC recommends inpatient therapy. Recommended parenteral regimes include the following:
References:
1. CDC Sexually Transmitted Disease Treatment Guidelines, 2010. Available at http://www.cdc.gov/std/treatment/2010/STD-Treatment-2010-RR5912.pdf. Accessed Feb. 9, 2012.
The CDC recommends empiric treatment for PID in sexually active young women and other women at risk for STDs if no cause for symptoms can be found and the patient has at least one of the following:
1. Cervical motion tenderness
OR
2. Adnexal tenderness
OR
3. Uterine tenderness
The following criteria enhance specificity and further support the diagnosis of PID:
1. Temp > 101 F
2. Abnormal mucopurulent cervical or vaginal discharge
3. Presence of abundant leukocytes on wet prep
4. Elevated ESR
5. Elevated CRP
6. Laboratory evidence of C. trachomatis or N. gonorrhea cervical infection
For patients with mild to moderate PID who can be managed as outpatients, the CDC recommends:
Patient who don't respond to oral therapy within 72 hours should be reevaluated both to confirm the diagnosis and to initiate parenteral therapy.
For patients with severe illness, tubo-ovarian abscess, pregnancy, or lack of response to oral agents, the CDC recommends inpatient therapy. Recommended parenteral regimes include the following:
References:
1. CDC Sexually Transmitted Disease Treatment Guidelines, 2010. Available at http://www.cdc.gov/std/treatment/2010/STD-Treatment-2010-RR5912.pdf. Accessed Feb. 9, 2012.
Thursday, January 26, 2012
Dog bites: When should you use prophylactic antibiotics?
Dog bites are the most common mammalian bite injury seen in the United States, followed by cat bites and human bites. Management of minor dog bite wounds includes copious irrigation with normal saline (>150 mL through an 18 or 19 gauge plastic catheter), debridement of any devitalized or crushed tissue, assessment for underlying tendon or bone injury, updating tetanus status when indicated, and assessment of rabies risk. Primary closure of bite wounds is controversial, but it is generally accepted that wounds can be sutured unless they are high risk or already infected. High risk wounds should be closed with delayed primary closure 72 hours later. Wounds to the face are usually sutured for optimal cosmetic results, and infection is rare due to copious irrigation, excellent blood supply, and use of antibiotic prophylaxis.
There is a lack of consensus about which type of wounds require antibiotic prophylaxis. Risk factors for infection include location on hand, foot, or over a major joint, puncture wounds and crush injuries, treatment delay > 12 hours, and systemic factors such as advanced age, immunosuppression, asplenism, diabetes, and vascular disease. Griego et al list the following indications for prophylactic antibiotics:
There is no clear consensus on the appropriate antibiotic choice for infection prophylaxis. When infections do develop from dog bites, they are usually polymicrobial with both aerobic and anaerobic species. Staphylococcus, Streptococcus, and Corynebacterium are the most common aerobic species isolated, with Bacteriodes fragilis, Prevotella, Peptostreptococcus, and Fusobacterium among common anaerobic isolates. A rare, but potentially fatal infection associated with dog bites is Capnocytophaga canimorsus. Patients who develop this infection usually have a predisposing condition, such as splenectomy.
Amoxicillin/clavulanate is often prescribed due its dual aerobic and anaerobic coverage. Alternative regimens include clindamycin plus ciprofloxacin, TMP/SMX, and second- or third-generation cephalosporins such as cefuroxime. Duration should be 5-7 days. It is recommended that patients have an initial follow-up visit at 24-48 hours to assess wound healing and presence of infection.
References:
1. Griego RD et al. Dog, cat, and human bite wounds: A review. J Am Acad Dermatol 1995;33:1019-29.
2. Smith PF, Meadowcroft AM, May DB. Treating mammalian bite wounds. Journal of Clinical Pharmacy & Therapeutics. 2000;25:85-99
There is a lack of consensus about which type of wounds require antibiotic prophylaxis. Risk factors for infection include location on hand, foot, or over a major joint, puncture wounds and crush injuries, treatment delay > 12 hours, and systemic factors such as advanced age, immunosuppression, asplenism, diabetes, and vascular disease. Griego et al list the following indications for prophylactic antibiotics:
There is no clear consensus on the appropriate antibiotic choice for infection prophylaxis. When infections do develop from dog bites, they are usually polymicrobial with both aerobic and anaerobic species. Staphylococcus, Streptococcus, and Corynebacterium are the most common aerobic species isolated, with Bacteriodes fragilis, Prevotella, Peptostreptococcus, and Fusobacterium among common anaerobic isolates. A rare, but potentially fatal infection associated with dog bites is Capnocytophaga canimorsus. Patients who develop this infection usually have a predisposing condition, such as splenectomy.
Amoxicillin/clavulanate is often prescribed due its dual aerobic and anaerobic coverage. Alternative regimens include clindamycin plus ciprofloxacin, TMP/SMX, and second- or third-generation cephalosporins such as cefuroxime. Duration should be 5-7 days. It is recommended that patients have an initial follow-up visit at 24-48 hours to assess wound healing and presence of infection.
References:
1. Griego RD et al. Dog, cat, and human bite wounds: A review. J Am Acad Dermatol 1995;33:1019-29.
2. Smith PF, Meadowcroft AM, May DB. Treating mammalian bite wounds. Journal of Clinical Pharmacy & Therapeutics. 2000;25:85-99
Monday, January 23, 2012
To bolus or not to bolus in DKA
 |
| IVF and insulin drip |
In children with diabetic ketoacidosis, an insulin bolus is NOT recommended. It is one of the factors that has been linked to increased risk of cerebral edema. Do not bolus insulin in kids with DKA.
In adults the guidelines are less clear. A recent review in Annals of Internal Medicine says that either a bolus followed by drip can be given, (regular insulin at 0.1 U/kg IV bolus followed by 0.1 U/kg/hr as continuous IV infusion), or simply an infusion started. Tintinalli recommends just starting insulin infusion at 0.1 U/kg/hr.
A few pearls:
1. Always give fluids before anything else, while awaiting lab results. The average adult with DKA has a water deficit of 5-10 L.
2. Wait for potassium level before starting insulin. Many patients with DKA have a profound potassium deficit, even though serum levels can initially be normal or even high. This is because the acidosis drives potassium out of cells in exchange for hydrogen ions, falsely elevating the potassium level. If initial [K+] level is <3.3, give potassium-containing fluids BEFORE starting insulin. Otherwise life-threatening hypokalemia can result.
3. Bicarbonate therapy is controversial due to risk of worsening hypokalemia, worsening intracellular acidosis. Bicarbonate should not be routinely given, but should be considered for severe acidosis (pH < 6.9).
4. Remember to look for precipitants of DKA, and treat any underlying conditions.
References:
1. Rosenbloom, AL. The management of diabetic ketoacidosis in children. Diabetes Therapy 2010. 1:103-120.
2. From Tintinalli's Emergency Medicine: A Comprehensive Study Guide, 7th Ed. Tintinalli, JE, Ed, et al. New York: McGraw Hill, 2011: p. 442-445.
3. Wilson et al. In clinic: Diabetic ketoacidosis. Annals Internal Medicine 2010. 152(1):ITC1-1.
The management of diabetic ketoacidosis in children.Source:Diabetes Therapy [1869-6953] Rosenbloom yr:2010 vol:1 iss:2 pg:103 -120
Sunday, January 15, 2012
Beta blocker overdose
 |
| Sinus bradycardia with HR of 50 |
The differential for bradycardia is broad and includes vasovagal response, sick sinus syndrome, myocardia ischemia involving right coronary artery, atrioventricular block, increased intracranial pressure (seen with elevated BP in Cushing's reflex), hypokalemia, hypothermia, hypothyroidism, and medications (beta blockers, calcium channel blockers, digoxin, clonidine). Bradycardia can also be a normal finding, often seen in well-conditioned athletes.
The key branch point for management of bradycardia is whether or not signs of adequate perfusion are present. If the bradycardia is leading to hypoperfusion, according to ACLS guidelines, transcutaneous pacing should be initiated while reversible causes (H's and T's) are investigated. If the bradycardia is not causing hemodynamic compromise, patients can be monitored and observed.
While transcutaneous pacing is being set up, atropine 0.5 mg IV push should be given (repeat for total dose of 3 mg). Dopamine is a second-line agent for when atropine is not effective. Dose is 2-10 mcg/kg/min infusion. Epinephrine is a third-line agent, dose 2-10 mcg/min infusion. If beta blocker overdose is suspected, glucagon 1-5 mg IV should be given. If heart rate responds, a glucagon infusion should be started.
Pacer pads were placed on the patient, but because she was maintaing her blood pressure and mentating well, transcutaneous pacing was not required. She was given a fluid bolus as well as atropine 0.5 mg IV x 2 doses without improvement. Because beta blocker overdose was a consideration, she was given glucagon 3.5 mg IV. Because of possible ischemic changes on EKG, an aspirin was administered and cardiology was consulted.
Tuesday, January 10, 2012
Causes of hypertensive emergency
Causes of hypertensive emergency
Hypertensive emergency is defined as BP > 220/140 in the presence of target-organ damage. Most cases of hypertensive emergency occur in patients with essential hypertension who have inadequate treatment of their hypertension or have discontinued their medications. However there are many other causes of hypertensive emergencies:
1. Renal parenchymal disease--acute glomerulonephritis, chronic pyelonephritis, tubulointerstitial nephritis
2. Renovascular disease--fibromuscular dysplasia, polyarteritis nodosa, atherosclerosis
3. Systemic disorders affecting kidneys-SLE, other vasculidities
4. Endocrine causes--pheochromocytoma, Cushing syndrome, primary hyperaldosteronism (Conn's syndrome when caused by adrenal adenoma)
5. Drugs--cocaine, amphetamine, ephedrine, diet pills, cyclosporine, clonidine withdrawal, oral contraceptives,
6. Drug interactions--Monamine oxidase inhibitors when combined with tricyclic antidepressants, antihistamines, or tyramine-containing foods
7. Pregnancy-related--preeclampsia/eclampsia
8. CNS causes--CNS trauma, subarachnoid hemorrhage, ischemic and hemorrhagic stroke, spinal cord disorders
9. Cardiovascular causes--coarctation of the aorta, acute aortic dissection
Classic teaching is that hypertensive emergency should be managed with IV agents to lower blood pressure by 20% within a few hours. However, Tintinalli recommends treatment for hypertensive emergency should be stratified by diagnosis. For example, in acute aortic dissection, SBP should be lowered to 100-120 range to reduce shearing forces on the vasculature. In intracranial hemorrhage, SBP goal is < 160 to reduce chance of rebleeding. In acute ischemic stroke, however, some degree of HTN is appropriate (maintain cerebral perfusion), but if fibrinolysis is planned, target BP is >185/110. In acute renal failure, blood pressure reduction by no more than 20% is advised. In acute sympathic crisis (cocaine, amphetamines, etc.), benzodiazepines can be administered for symptom relief.
References:
1. Riaz K et al. Emedicine: Hypertension Treatment & Management. Available at http://emedicine.medscape.com/article/241381-overview. Accessed Jan. 10, 2012.
2. From Tintinalli's Emergency Medicine: A Comprehensive Study Guide, 7th Ed. Tintinalli, JE, Ed, et al. New York: McGraw Hill, 2011: p. 442-445.
Hypertensive emergency is defined as BP > 220/140 in the presence of target-organ damage. Most cases of hypertensive emergency occur in patients with essential hypertension who have inadequate treatment of their hypertension or have discontinued their medications. However there are many other causes of hypertensive emergencies:
1. Renal parenchymal disease--acute glomerulonephritis, chronic pyelonephritis, tubulointerstitial nephritis
2. Renovascular disease--fibromuscular dysplasia, polyarteritis nodosa, atherosclerosis
3. Systemic disorders affecting kidneys-SLE, other vasculidities
4. Endocrine causes--pheochromocytoma, Cushing syndrome, primary hyperaldosteronism (Conn's syndrome when caused by adrenal adenoma)
5. Drugs--cocaine, amphetamine, ephedrine, diet pills, cyclosporine, clonidine withdrawal, oral contraceptives,
6. Drug interactions--Monamine oxidase inhibitors when combined with tricyclic antidepressants, antihistamines, or tyramine-containing foods
7. Pregnancy-related--preeclampsia/eclampsia
8. CNS causes--CNS trauma, subarachnoid hemorrhage, ischemic and hemorrhagic stroke, spinal cord disorders
9. Cardiovascular causes--coarctation of the aorta, acute aortic dissection
Classic teaching is that hypertensive emergency should be managed with IV agents to lower blood pressure by 20% within a few hours. However, Tintinalli recommends treatment for hypertensive emergency should be stratified by diagnosis. For example, in acute aortic dissection, SBP should be lowered to 100-120 range to reduce shearing forces on the vasculature. In intracranial hemorrhage, SBP goal is < 160 to reduce chance of rebleeding. In acute ischemic stroke, however, some degree of HTN is appropriate (maintain cerebral perfusion), but if fibrinolysis is planned, target BP is >185/110. In acute renal failure, blood pressure reduction by no more than 20% is advised. In acute sympathic crisis (cocaine, amphetamines, etc.), benzodiazepines can be administered for symptom relief.
References:
1. Riaz K et al. Emedicine: Hypertension Treatment & Management. Available at http://emedicine.medscape.com/article/241381-overview. Accessed Jan. 10, 2012.
2. From Tintinalli's Emergency Medicine: A Comprehensive Study Guide, 7th Ed. Tintinalli, JE, Ed, et al. New York: McGraw Hill, 2011: p. 442-445.
Saturday, January 7, 2012
Neonatal sepsis
Question: What antibiotics should be given for a newborn with suspected septic shock?
Ampicillin PLUS gentamicin OR cefotaxime (from Tintinalli)
The main organisms to be concerned about are gram-positives (mainly Group B strep, also Listeria) and gram-negatives (E. coli). Ampicillin, an extended-spectrum penicillin, covers group B strep and Listeria, while gentamicin, an aminoglycoside, provides coverage for gram negative organisms. If gram negative meningitis is suspected, cefotaxime is favored for its better CNS penetration.
More on neonatal sepsis:
In the critically ill neonate, the diagnosis of sepsis should always be considered. In the ED setting, it is not always possible to distinguish the various causes of the neonate in distress--septic shock, cardiogenic shock from congenital heart disease, inborn errors of metabolism, etc. Therefore empiric treatment for septic shock should be initiated in the critically ill newborn, as early treatment of sepsis has been shown to improve outcomes.
Early-onset sepsis
Early-onset sepsis (within first few days of life) is typically due to infection acquired from the mother, either transplacentally, transcervically, or via contact with microbe during passage through a colonized birth canal. Organisms responsible for early-onset sepsis include:
1. Group B Strep
2. E. coli
3. Coagulase negative staph
4. Haemophilus influenzae
5. Listeria monocytogenes
Risk factors for early-onset neonatal sepsis include maternal GBS colonization (though incidence of GBS infection has been reduced due to third-trimester screening programs), premature rupture of membranes, prolonged rupture of membranes, preterm rupture of membranes, chorioamnionitis, prematurity, and maternal UTI.
Workup for the neonate with suspected sepsis includes CBC with differential, blood cultures, CSF gram stain and culture, chest xray, urine studies, and possibly CRP or other infection markers. Hemodynamic support should be provided with IV fluids and pressors, if indicated. Antibiotics should be started empirically to cover for gram positive organisms (especially group B strep), and gram-negative organisms (namely E. coli). For infants < 1 week of age, Tintinalli recommends ampicillin PLUS gentamicin OR cefotaxime.
A 2009 Cochrane review sought to compare the efficacy of different antibiotic regimens for neonatal sepsis, and found that there was no evidence to recommend one particular regimen over another. This was largely due to small sample size (only 2 studies comparing treatment of sepsis in infants < 48 hours old).
Late-onset sepsis
For infants age 1-4 weeks, Tintinalli recommends ampicillin PLUS cefotaxime/ceftiaxone. This covers for gram positive and gram negative organisms.
References:
1. From Tintinalli's Emergency Medicine: A Comprehensive Study Guide, 7th Ed. Tintinalli, JE, Ed, et al. New York: McGraw Hill, 2011: p. 74, 1012.
2. Cochrane review. Antibiotics regimens for suspected early neonatal sepsis. Published online 21 Jan 2009. Available at http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004495.pub2/abstract. Accessed Jan 7. 2012.
Ampicillin PLUS gentamicin OR cefotaxime (from Tintinalli)
The main organisms to be concerned about are gram-positives (mainly Group B strep, also Listeria) and gram-negatives (E. coli). Ampicillin, an extended-spectrum penicillin, covers group B strep and Listeria, while gentamicin, an aminoglycoside, provides coverage for gram negative organisms. If gram negative meningitis is suspected, cefotaxime is favored for its better CNS penetration.
More on neonatal sepsis:
In the critically ill neonate, the diagnosis of sepsis should always be considered. In the ED setting, it is not always possible to distinguish the various causes of the neonate in distress--septic shock, cardiogenic shock from congenital heart disease, inborn errors of metabolism, etc. Therefore empiric treatment for septic shock should be initiated in the critically ill newborn, as early treatment of sepsis has been shown to improve outcomes.
Early-onset sepsis
Early-onset sepsis (within first few days of life) is typically due to infection acquired from the mother, either transplacentally, transcervically, or via contact with microbe during passage through a colonized birth canal. Organisms responsible for early-onset sepsis include:
1. Group B Strep
2. E. coli
3. Coagulase negative staph
4. Haemophilus influenzae
5. Listeria monocytogenes
Risk factors for early-onset neonatal sepsis include maternal GBS colonization (though incidence of GBS infection has been reduced due to third-trimester screening programs), premature rupture of membranes, prolonged rupture of membranes, preterm rupture of membranes, chorioamnionitis, prematurity, and maternal UTI.
Workup for the neonate with suspected sepsis includes CBC with differential, blood cultures, CSF gram stain and culture, chest xray, urine studies, and possibly CRP or other infection markers. Hemodynamic support should be provided with IV fluids and pressors, if indicated. Antibiotics should be started empirically to cover for gram positive organisms (especially group B strep), and gram-negative organisms (namely E. coli). For infants < 1 week of age, Tintinalli recommends ampicillin PLUS gentamicin OR cefotaxime.
A 2009 Cochrane review sought to compare the efficacy of different antibiotic regimens for neonatal sepsis, and found that there was no evidence to recommend one particular regimen over another. This was largely due to small sample size (only 2 studies comparing treatment of sepsis in infants < 48 hours old).
Late-onset sepsis
Late-onset sepsis (between 4-90 days of life) is more likely due to organisms acquired from the caregiving environment (including hospital-acquired infections). While pneumonia is more common in early-onset sepsis, meningitis and bacteremia are more common in late-onset sepsis. Organisms include:
1. Coagulase negative staph
2. Staph aureus
3. E. coli
4. Klebsiella
5. Pseudomonas
6. Group B strep
7. Strep pneumoniaeFor infants age 1-4 weeks, Tintinalli recommends ampicillin PLUS cefotaxime/ceftiaxone. This covers for gram positive and gram negative organisms.
References:
1. From Tintinalli's Emergency Medicine: A Comprehensive Study Guide, 7th Ed. Tintinalli, JE, Ed, et al. New York: McGraw Hill, 2011: p. 74, 1012.
2. Cochrane review. Antibiotics regimens for suspected early neonatal sepsis. Published online 21 Jan 2009. Available at http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004495.pub2/abstract. Accessed Jan 7. 2012.
Thursday, January 5, 2012
Diagnosis of meningitis
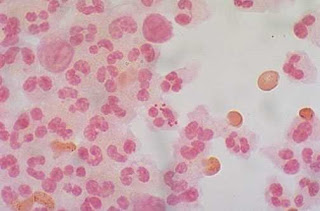 |
| Figure 1: Neisseria meningitidis, often referred to as meningococcus |
Diagnosis of meningitis in adults: Sensitivity of physical exam
Meningitis is a difficult-to-diagnose disease with potentially devastating consequences. Meningitis refers to inflammation (usually infectious) of the meninges, a layer of membranes that enclose the brain and spinal cord. Meningitis can be caused by bacteria, viruses, and fungi. Common causes of bacterial meningitis include Strep pneumoniae (most common bacterial cause), Hemophilus influenza, Staphylococcus aureus, Neisseria meningitidis, and Mycobacterium tuberculosis. Meningitis typically results from hematogenous spread of bacteria to seed the meninges. However, it can also occur from direct extension from sinusitis or otitis media, following neurosurgery, skull fracture, or other trauma which create a portal of entry of organisms. Without treatment bacterial meningitis is usually fatal, and even with treatment, the mortality rate is 25%.
Other causes of meningitis include viral (enterovirus, HSV), fungal (cryptococcus), and parasitic. Aspetic meningitis refers to a category in which meningeal inflammation is present but no infectious organism is identified. Most of these cases are likely viral, but partially treated bacterial meningitis can also masquerade as aseptic meningitis.
Because of its nonspecific signs and symptoms, meningitis is a notoriously difficult disease to diagnose. Physical exam findings such as Kernig's signs, Brudzinski's signs, and nuchal rigidity can aid in the diagnosis, but lack sensitivity and specificity.
A 2002 study in Clinical Infectious Disease evaluated the sensitivity of these bedside tests. 297 patients with suspected meningitis were examined prior to undergoing lumbar puncture to evaluate for meningitis (defined as >6 WBCs/mL spinal fluid). The researchers found that the sensitivities for Kernig's sign, Brudzinski's sign, and nuchal rigidity for meningitis were a dismal 5%, 5%, and 30%, respectively. In the very small subset of patients with severe meningeal irritation (4 patients; defined as >1000 WBCs/mL spinal fluid), only nuchal rigidity, but not the other two signs, had diagnostic value with a sensitivity of 100%. The conclusion of the study was that these bedside tests are "too insensitive to identify the majority of patients with meningitis in contemporary practice."
Why am I writing this post? Because I recently had a patient with fever and a headache. She had no neck stiffness and no nuchal rigidity on exam. In my mind this put the diagnosis of meningitis lower on the differential, which led to a delay in her getting a lumbar puncture, which, as it turns out, showed she did in fact have meningitis. Fortunately the the patient did well. But it was an important learning case for me. If the sensitivity of nuchal rigidity is only 30% as this study reports (similar data seen in other studies), clearly I should not have felt reassured by her lack of nuchal rigidity, any more than I would have determined that a patient with a negative psoas sign could not have appendicitis.
No single physical exam finding or historical feature can rule in, or rule out, the diagnosis of meningitis. The gold standard for diagnosis, a lumbar puncture, is a test with costs both to patient and physician, and not every patient with fever and headache should get a lumbar puncture. This would expose patients to unnecessary risks and discomfort and create a disaster of work flow in a busy ED. Rather the entire picture needs to be taken into account to push one to do the lumbar puncture: historical features, patient appearance and physical exam findings, input from family/friends, and ultimately physician "gestalt." This is what makes our job as emergency physicians challenging, and yet fun. There are no algorithms or easy formulas to apply to evasive diagnoses like meningitis. Knowing what to do when is the part of the art of medicine, something we all are all perfecting in our practice.
References:
1. Razonable, R, Cunha B. Meningitis. Available at http://emedicine.medscape.com/article/232915-overview. Accessed January 5, 2012.
2. Thomas KE, Hasbun R, Jekel J, ad Quagliarella VJ. The diagnositc accuracy of Kernig’s sign, Brudzinski’s sign, and nuchal rigidity in adults with suspected meningitis. Clin Infect Dis 2002. 35:46-52.
Subscribe to:
Comments (Atom)







